Chemistry Atomic Structure Exam Questions
Atomic Structure Practice Exam Questions
Atomic Structure explains what substances are made of, how elements are organised, and how different materials behave.
Download and print our practice exam questions on the chemistry topic of atomic structure.
Learn more

Visit our Atomic Structure page for revision notes and videos to help you study.
Atomic Structure Practice Exam Worksheet
Practice exam style questions on atomic structure.
Answers below.
Click to reveal answers
| Question | Answer | Extra information |
| 1 | B [1 mark] | |
| 2 | D [1 mark] | |
| 3 | E [1 mark] | |
| 4 | C [1 mark] | |
| 5 | 92.5 x 6 and 7x 7.5 [1 mark] 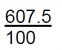 [1 mark] [1 mark]
6.075 [1 mark] 6.08 [1 mark] |
allow 6.08 with no working shown for 4 marks |
| 6 | any one from:
heat stir [1 mark] |
|
| 7 | filter [1 mark] | accept use a centrifuge accept leave longer (to settle) |
| 8 | any one from:
wear safety spectacles/googles wear an apron [1 mark] |
|
| 9 | evaporation at A [1 mark]
condensation at B [1 mark] |
|
| 10 | 100 [1 mark] | |
| 11 | electrons transferred from potassium to sulfur [1 mark]
two potassium atoms each lose one electron [1 mark]
sulfur atoms gain 2 electrons [1 mark]
|
|
| 12 | there are no gaps/sticks between the potassium ions and sulfide ions [1 mark] | |
| 13 | 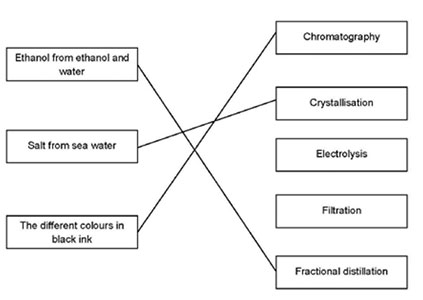 [3 marks] [3 marks] |
for every correct line [1 mark] |
| 14 | include a (filter) funnel [1 mark] | allow funnel drawn on the diagram ignore clamp stand |
| 15 | evaporate [1 mark]
condense [1 mark] |
must be in this order |
| 16 | nucleus [1 mark] neutron [1 mark] neutron[1 mark] electron[1 mark] proton[1 mark] |
must be in this order |
| 17 | 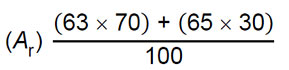 [1 mark] [1 mark]
= 63.6 [1 mark] |
an answer of 63.6 scores [2 marks] |
| 18 | 26 [1 mark] 30 [1 mark] 26 [1 mark] |
must be in this order |

