
Crude Oil & Fuels
Crude oil plays a vital role in modern life, and understanding its chemistry is essential for GCSE Chemistry. This topic explores what crude oil is made of, how it's processed, and why it’s so important to everyday life.
What is Crude Oil?
Crude oil is a fossil fuel formed from the remains of ancient marine organisms, buried under layers of rock for millions of years. It’s a mixture of hydrocarbons — compounds made up only of hydrogen and carbon atoms.
Hydrocarbons and Alkanes
The hydrocarbons found in crude oil come in many shapes and sizes. One key type is the alkanes, which are saturated hydrocarbons. This means they only contain single bonds between carbon atoms.
General formula for alkanes:
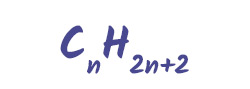
Examples include:
- Methane (CH₄)
- Ethane (C₂H₆)
- Propane (C₃H₈)
As you go up the series, the boiling point increases and the flammability decreases.
Complete Combustion of Hydrocarbons
When alkanes burn in plenty of oxygen, they undergo complete combustion, producing:
- Carbon dioxide (CO₂)
- Water (H₂O)
- And releasing energy as heat and light
Equation Example:

This is an exothermic reaction — it gives out energy.
Revision Notes

The Cornell method is like a supercharged note-taking system that helps you ace your revision!
Print out our blank revision notes pages to help you revise.
How to make effective revision notes with the Cornell method.
Exam Questions & Answers

Download and print off practice our FREE worksheet with exam style questions on Cell Biology.
Fractional Distillation of Crude Oil
Crude oil is separated into useful substances using fractional distillation. This method works because hydrocarbons have different boiling points.
The crude oil is:
- Heated until it vaporises.
- The vapour enters a fractionating column which is hotter at the bottom and cooler at the top.
- Different hydrocarbons condense at different heights depending on their boiling points.
Fractions include:
- Refinery gases (used for cooking)
- Petrol (car fuel)
- Kerosene (jet fuel)
- Diesel, fuel oil, and bitumen
Uses of Crude Oil
Crude oil is used to make:
- Fuels: petrol, diesel, kerosene
- Plastics and polymers
- Lubricants and solvents
It’s also the raw material for many chemicals used in medicine, agriculture, and manufacturing.
Cracking Crude Oil
Not all hydrocarbons from crude oil are equally useful. Long-chain hydrocarbons are less flammable and harder to vaporise. These are broken down in a process called cracking.
There are two main types:
- Thermal cracking (high temperature and pressure)
- Catalytic cracking (using a catalyst)
Cracking produces:
- Shorter alkanes (useful as fuels)
- Alkenes (used to make plastics)
Example Reaction:
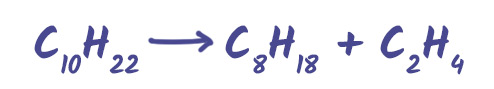
This helps meet demand for fuels and makes raw materials for new products.
Also see Organic Reactions, Polymers, Chemical Analysis
Revision Notes

The Cornell method is like a supercharged note-taking system that helps you ace your revision!
Print out our blank revision notes pages to help you revise.
How to make effective revision notes with the Cornell method.
Why Do I Need to Know About Crude Oil & Fuels?
In Everyday Life
- From the petrol in cars to the gas heating your home, crude oil products keep the world moving.
- Many items you use daily, like plastic bottles, clothes, packaging, and even cosmetics, come from hydrocarbons.
- Learning about combustion helps you understand issues like climate change, carbon emissions, and air quality.
In Science & Chemistry Careers
- Applies key chemical principles like boiling points, molecular structure, and reactions.
- Teaches separation techniques such as fractional distillation and cracking, which link to industrial chemistry.
- Develops equation skills with combustion and cracking reactions, and reinforces the importance of balancing equations.


