Electrolysis
Electrolysis is a key chemical process used to break down substances using electricity. It’s important in industry for extracting reactive metals and purifying materials, and it also helps us understand how ions behave when electricity is passed through solutions.
Practicals on this page:
Electrolysis
What Is Electrolysis?
Electrolysis is the breakdown of an ionic compound using electricity.
It only works when:
- The compound is molten (melted) or dissolved in water (aqueous)
- Ions are free to move and carry the electric current
During electrolysis:
- Positive ions (cations) move to the negative electrode (cathode)
- Negative ions (anions) move to the positive electrode (anode)
This movement of ions allows chemical changes to occur at each electrode.
Electrolysis of Molten Ionic Compounds
When ionic compounds are molten, they contain only the metal and non-metal ions.
Example:
Molten lead bromide (PbBr₂)
- Pb²⁺ goes to the cathode → gains electrons → lead metal forms
- Br⁻ goes to the anode → loses electrons → bromine gas forms
This type of electrolysis is used in industry to extract reactive metals that can’t be reduced with carbon.
Extracting Metals Using Electrolysis
Metals that are more reactive than carbon (e.g. aluminium, potassium) must be extracted by electrolysis.
Example: Aluminium extraction from bauxite (aluminium oxide)
Aluminium oxide is dissolved in molten cryolite to lower the melting point
Electrolysis separates aluminium (at the cathode) and oxygen gas (at the anode)
This process requires a lot of energy, making it expensive.
Revision Notes

The Cornell method is like a supercharged note-taking system that helps you ace your revision!
Print out our blank revision notes pages to help you revise.
How to make effective revision notes with the Cornell method.
Exam Questions & Answers

Download and print off practice our FREE worksheet with exam style questions on Cell Biology.
Electrolysis of Aqueous Solutions
Aqueous solutions contain ions from the compound and ions from water (H⁺ and OH⁻).
This means more than one possible product can form at each electrode.
At the cathode (where reduction happens):
- If the metal is more reactive than hydrogen, hydrogen gas is produced
- If the metal is less reactive than hydrogen, the metal is deposited
At the anode (where oxidation happens):
- If the solution contains halide ions (Cl⁻, Br⁻, I⁻), the halogen is produced
- If no halide ions are present, oxygen gas is released from OH⁻
Examples of Electrolysis of Aqueous Solutions
Sodium chloride (NaCl) solution:
- Cathode: H⁺ → H₂ gas (Na is more reactive than hydrogen)
- Anode: Cl⁻ → Cl₂ gas (a halide is present)
Copper sulfate (CuSO₄) solution:
- Cathode: Cu²⁺ → Copper metal (less reactive than hydrogen)
- Anode: No halides, so OH⁻ → O₂ gas
Half Equations
Half equations show what happens to the ions at each electrode during electrolysis. They include the gain or loss of electrons.
Reduction (gain of electrons) – occurs at the cathode
Example:
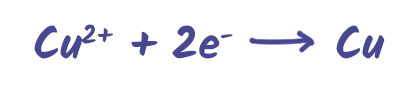
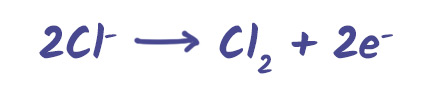
Oxidation (loss of electrons) – occurs at the anode
Example:
Writing half equations helps you understand the electron transfer in redox reactions.
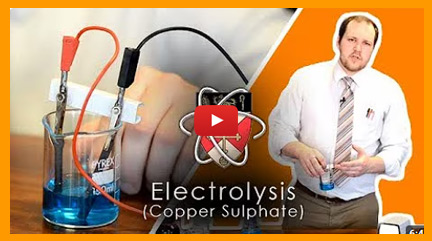
PRACTICAL - Electrolysis
In this practical, you investigate the products formed when an aqueous ionic solution is electrolysed using inert electrodes (like graphite or carbon rods).
A common example is the electrolysis of copper(II) chloride solution:
- At the cathode (negative electrode): copper metal is deposited
- At the anode (positive electrode): chlorine gas is produced
You’ll:
- Set up the circuit with a power supply and two inert electrodes
- Place them into the electrolyte solution
- Collect and observe the products at each electrode (e.g. bubbles, solid deposits)
This helps you understand ion movement, displacement, and how to identify products based on the reactivity series and presence of halide ions.
Revision Notes

The Cornell method is like a supercharged note-taking system that helps you ace your revision!
Print out our blank revision notes pages to help you revise.
How to make effective revision notes with the Cornell method.
Why Do I Need to Know About Electrolysis?
In Everyday Life
- Understanding how batteries and fuel cells work
- Learning how aluminium and copper are made for cans, cables, or cookware
- Realising how clean hydrogen can be used for energy in the future
- Seeing how wastewater is treated to protect health
- Appreciating the chemistry behind electroplating jewellery or tools
In Science & Chemistry Careers
- Extracting and purifying metals from ores
- Using half equations to understand redox reactions
- Developing renewable energy technologies, like hydrogen fuel cells
- Applying electrolysis in chemical manufacturing, electronics, or nanotech
- Working in environmental science, engineering, or materials science