
Heating
When things get heated up, energy moves from one place to another. For example, when you boil water on a stove, the heat from the stove transfers to the water, making it hot. This transfer of energy is like passing a "hot potato" from one object to another.
Equations on this page:
Efficiency
Practicals on this page:
Thermal Insulators and Specific Heat Capacity.
What is conduction and convection?
Conduction is a process where heat energy is transferred through a material or between materials that are in direct contact. Imagine you're holding a metal spoon in a hot bowl of soup. As the spoon touches the hot soup, heat energy flows from the soup to the spoon through direct contact. The particles in the spoon start to vibrate more vigorously, transferring heat energy to neighboring particles until the entire spoon becomes hot.
Convection is a process where heat energy is transferred through the movement of fluids (liquids or gases). An example of this is how air moves around a room when you use a heater. The heater warms the air near it, causing it to rise. As the warm air rises, cooler air moves in to take its place, creating a convection current that spreads heat around the room.
What is Lubrication and Insulation?
Lubrication is a process of reducing friction between surfaces that are in contact with each other. Friction is the force that opposes motion when two surfaces rub against each other. Lubricants are substances used to reduce this friction and make the surfaces slide more easily.
The chain on your bicycle needs lubrication to move smoothly and efficiently. Without lubrication, the chain would create a lot of friction against the gears, making it harder for you to pedal. Lubricants like oil or grease are applied to the chain to reduce friction, allowing it to move more freely.
Insulation is a material or substance used to prevent the transfer of heat, electricity, or sound. It acts as a barrier that slows down or blocks the flow of these forms of energy.
Imagine you're wearing a winter jacket on a cold day. The jacket has layers of insulation, such as down feathers or synthetic fibers, that trap heat close to your body and prevent it from escaping. This keeps you warm by reducing heat loss to the surrounding environment.
Similarly, in a house, insulation is added to walls, floors, and ceilings to keep the indoor temperature comfortable and to reduce energy costs by preventing heat from escaping during the winter or entering during the summer.
Reducing unwanted energy transfers
Both lubrication and insulation are ways to reduce the amount of energy that get transferred to un-useful stores. By implementing these methods, we can reduce energy wastage, improve efficiency, and conserve resources.
Key Words
Thermal conductivity - measure of how quickly energy is transferred through a material.
Convection currents - the cycle of warm air rising and cool air sinks.
Also see Energy, Energy resources
Revision Notes

The Cornell method is like a supercharged note-taking system that helps you ace your revision!
Print out our blank revision notes pages to help you revise.
How to make effective revision notes with the Cornell method.
Exam Questions & Answers

Download and print off practice our FREE worksheet with exam style questions on Cell Biology.
PRACTICAL Thermal Insulators
This practical helps you investigate how well different materials reduce heat loss — in other words, how good they are as thermal insulators. You’ll compare materials by wrapping them around containers of hot water and measuring how quickly the water cools over time.
You might also test how thickness or the number of layers affects insulation. By recording the temperature at regular intervals, you can find out which material keeps the water warm the longest — meaning it’s the best insulator.
PRACTICAL Specific Heat Capacity
In this practical, you measure how much energy is needed to increase the temperature of a material. This helps you calculate its specific heat capacity — the amount of energy required to raise 1 kg of a substance by 1°C.
You’ll heat a metal block using an electric heater, measure the temperature change over time, and use the energy input (from a joulemeter or power × time) to calculate the value using the equation:
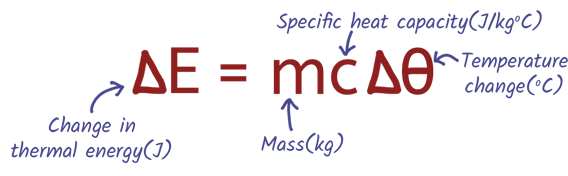
Links for Learning
Bitesize: Energy and heating
Pass my exams: Heat Energy and Heat Transfer
Revision Notes

The Cornell method is like a supercharged note-taking system that helps you ace your revision!
Print out our blank revision notes pages to help you revise.
How to make effective revision notes with the Cornell method.
Why Do I Need to Know About Heating?
In Everyday Life
- Understanding how insulation keeps homes warm and reduces energy bills
- Knowing why metals feel colder than wood, even at the same temperature
- Making better choices about energy-efficient appliances (like kettles or radiators)
- Understanding how heat spreads through cooking, heating systems, or even your clothes
- Seeing why energy is "wasted" as heat and how to reduce that loss in daily life
In Science & Physics Careers
- Designing better insulation materials for buildings, vehicles, or spacecraft
- Improving energy efficiency in heating systems, electronics, and engines
- Working in renewable energy (like solar thermal panels or heat pumps)
- Analysing thermal properties in product design, engineering, or materials science
- Using heat transfer knowledge in medicine (like body temperature control or thermal imaging)




