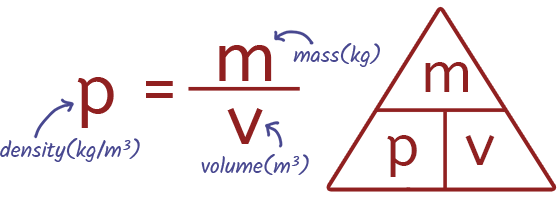Molecules and Matter
Molecules are tiny particles that make up all matter around us, like water, air, and even ourselves. They're made of atoms bonded together, and how they're arranged determines the properties of different materials, like their shape, size, and behaviour. Understanding molecules helps us grasp how substances interact and why matter behaves the way it does.
Equations on this page:
Density and Specific Latent Heat
Solids, liquids and gases
There are three main states of matter:
Solids have a definite shape and volume, meaning they maintain their shape and size regardless of the container they are placed in. Particles in solids are closely packed together and vibrate in fixed positions.
Liquids have a definite volume but take the shape of their container. They flow and can be poured, but they don't expand to fill the entire container like gases do. Particles in liquids are close together but can move past each other, allowing liquids to flow.
Gases have neither a definite shape nor volume and completely fill the space available to them. They can be compressed into a smaller volume. Particles in gases are far apart and move freely, bouncing off each other and the walls of the container.
What is internal energy?
Internal energy is the total energy stored within a substance due to the motion and arrangement of its particles, including kinetic energy (energy of motion) and potential energy (energy stored in the bonds between particles). It's like the energy that's "hidden" inside a substance because of how its particles move and interact with each other.
Exam Questions & Answers

Download and print off practice our FREE worksheet with exam style questions on Molecules and Matter.
Change of state
Changes of state refer to the physical transformations that occur when matter transitions between different states, such as from a solid to a liquid or from a liquid to a gas. The number of particles doesn't change in any of the states, they are just arranged differently.
- Melting (Solid to Liquid): Melting is the process where a solid substance changes into a liquid state. This occurs when heat energy is added to the solid, causing its particles to gain energy and vibrate more vigorously. As the particles move more freely, the forces holding them in a fixed arrangement weaken, and the solid structure breaks down, resulting in the formation of a liquid. For example, when you heat an ice cube, it melts into liquid water.
- Freezing (Liquid to Solid): Freezing is the opposite of melting and occurs when a liquid substance changes into a solid state. This happens when heat energy is removed from the liquid, causing its particles to lose energy and slow down. As the particles lose energy, they become locked into a fixed arrangement, forming a solid structure. For example, when you put water in a freezer, it freezes into ice.
- Evaporation (Liquid to Gas): Evaporation is the process where a liquid substance changes into a gas state. This occurs when heat energy is added to the liquid, causing its particles to gain enough energy to escape from the surface and become gas particles. Evaporation can happen at any temperature, but it occurs more quickly at higher temperatures. For example, when you leave a puddle of water in the sun, it eventually evaporates into water vapor.
- Condensation (Gas to Liquid): Condensation is the opposite of evaporation and occurs when a gas substance changes into a liquid state. This happens when heat energy is removed from the gas, causing its particles to lose energy and come closer together. As the particles lose energy, they form liquid droplets. For example, when water vapor in the air comes into contact with a cold surface, like a glass of cold water, it condenses into liquid water droplets on the surface.
- Sublimation (Solid to Gas): Sublimation is the process where a solid substance changes directly into a gas state without passing through the liquid phase. This occurs when the solid's vapour pressure exceeds atmospheric pressure at a certain temperature, allowing the solid particles to escape directly into the gas phase. For example, dry ice (solid carbon dioxide) sublimes when exposed to room temperature air.
EQUATION Density
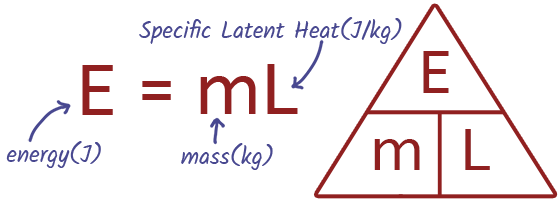
EQUATION Specific Latent Heat
Specific latent heat refers to the amount of heat energy required to change the state of 1kg of a substance without changing its temperature. There are two specific latent heats:
Specific Latent Heat of Fusion is the amount of heat energy required to change 1kg of a substance from solid to liquid at its melting point.
Specific Latent Heat of Vaporization is the amount of heat energy required to change 1kg of a substance from liquid to gas at its boiling point.
Revision Notes

The Cornell method is like a supercharged note-taking system that helps you ace your revision!
Print out our blank revision notes pages to help you revise.
How to make effective revision notes with the Cornell method.
Why Do I Need to Know About Molecules & Matter?
In Everyday Life
- Understanding what the states of matter (solid, liquid, gas) mean in the world around you
- Knowing how temperature and pressure affect gas behaviour—useful for weather, cooking, inflating tires, or breathing gear
- Learning why substances expand when heated and contract when cooled—like why rails have gaps and why mercury thermometers rise
- Grasping how gases exert pressure, why bubbles form or pop, and how this applies to things like soda cans or breathing systems
- Becoming aware of density, buoyancy, and how mass and volume affect whether objects float or sink
In Science & Physics Careers
- Analysing the behaviour of gases, liquids, and solids in engineering, materials science, and geophysics
- Designing systems that rely on pressure and temperature changes—like steam turbines, refrigeration, or air conditioning
- Working with fluid dynamics in aerospace, weather forecasting, and biological systems (like blood flow)
- Understanding phase changes and thermal properties in fields such as manufacturing, energy engineering, or environmental science
- Applying molecular and matter principles when developing new materials, thermal insulators, or energy-efficient technologies