Atomic Structure
Understanding atomic structure is the foundation of chemistry. It explains what substances are made of, how elements are organised, and how different materials behave. This topic covers atoms, the development of atomic theory, and how substances combine and separate.
Practicals on this page:
Separating Mixtures, Chromatography, Distillation
Atoms, Elements, and Compounds
 Everything around us is made of atoms, the smallest particles of an element that can exist. An atom has a tiny, dense nucleus in the centre, containing protons and neutrons, surrounded by electrons in shells (energy levels).
Everything around us is made of atoms, the smallest particles of an element that can exist. An atom has a tiny, dense nucleus in the centre, containing protons and neutrons, surrounded by electrons in shells (energy levels).
- Protons: Positive charge (+1), found in the nucleus.
- Neutrons: No charge (neutral), also in the nucleus.
- Electrons: Negative charge (–1), orbit in shells around the nucleus.
 Atoms of the same element all have the same number of protons. The number of protons is called the atomic number. The mass number is the total number of protons and neutrons.
Atoms of the same element all have the same number of protons. The number of protons is called the atomic number. The mass number is the total number of protons and neutrons.
An element is a substance made of only one type of atom. There are around 100 different elements, shown on the Periodic Table.
When atoms of different elements react together, they form compounds. Compounds contain atoms that are chemically bonded in fixed ratios.
Exam Questions & Answers

Download and print off practice our FREE worksheet with exam style questions on Atomic Structure.
Isotopes
Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. This means they have the same atomic number but different mass numbers. For example, carbon-12 and carbon-14 are both isotopes of carbon.

Some isotopes are stable, while others are radioactive and used in medical and scientific applications.
Chemical Equations
Chemical reactions can be shown using chemical equations. These show the reactants (what you start with) and the products (what you end with).
Word equations use names of substances, while symbol equations use chemical formulas.
Example:

Symbol:

To follow the law of conservation of mass, the number of atoms on both sides of the equation must be the same. This is called a balanced equation.
Mixtures and Separation Techniques
A mixture is made from two or more substances that are not chemically bonded. They can be physically separated using various methods.
Filtration
 Used to separate an insoluble solid from a liquid (e.g. sand from water).
Used to separate an insoluble solid from a liquid (e.g. sand from water).
Evaporation
 Used to separate a soluble solid from a liquid by heating the solution so the liquid evaporates (e.g. salt from saltwater).
Used to separate a soluble solid from a liquid by heating the solution so the liquid evaporates (e.g. salt from saltwater).
Crystallisation
Similar to evaporation but slower. The solution is gently heated to evaporate some water, then left to form crystals as it cools.
Distillation
Used to separate a liquid from a solution by heating it until it boils and collecting the condensed vapour. Simple distillation separates one liquid; fractional distillation is used to separate mixtures of liquids with different boiling points.
Chromatography
Used to separate mixtures of dyes or pigments. A spot of the sample is placed on chromatography paper and placed in a solvent. The solvent carries the substances at different speeds, separating them. Rf values can be used to identify substances.
History of the Atom
Our understanding of the atom has developed over time:
- John Dalton (early 1800s): Proposed atoms were tiny solid spheres.
- J.J. Thomson (1897): Discovered the electron; developed the plum pudding model — a ball of positive charge with negative electrons inside.
- Ernest Rutherford (1909): His alpha particle scattering experiment showed that atoms have a small, dense, positive nucleus — this led to the nuclear model of the atom.
- Niels Bohr (1913): Proposed that electrons orbit the nucleus in fixed energy levels or shells, which matched experimental evidence.
- Later discoveries showed that the nucleus contains protons and neutrons.
This progression helped shape the modern atomic model.
Electronic Structure
Electrons are arranged in energy levels (shells) around the nucleus. Each shell can hold a maximum number of electrons:
- 1st shell: up to 2 electrons
- 2nd shell: up to 8 electrons
- 3rd shell: up to 8 electrons (at GCSE level)
Electronic Structure Rules
Electrons fill the lowest energy levels first (starting closest to the nucleus).
Each shell must be full before electrons start filling the next one.
The number of electrons in the outer shell determines the atom’s reactivity and its group in the Periodic Table.
Example:
Oxygen (atomic number 8) has 8 electrons. Its electronic structure is 2,6.
PRACTICAL - Separating Mixtures
In chemistry, mixtures can be separated using physical methods because the substances are not chemically bonded. Three common techniques you need to know are filtration, evaporation, and crystallisation.
Filtration
Filtration is used to separate an insoluble solid from a liquid. The mixture is poured through filter paper — the solid stays on the paper (residue), and the liquid passes through (filtrate).
Evaporation
Evaporation separates a soluble solid from a solution. The solution is gently heated until the liquid evaporates, leaving the solid behind. This works well for things like salt from saltwater.
Crystallisation
Crystallisation is a slower method used to get pure, solid crystals from a solution. The solution is partially evaporated (to make it more concentrated), then left to cool slowly. Crystals form as the solubility of the solid decreases with cooling.
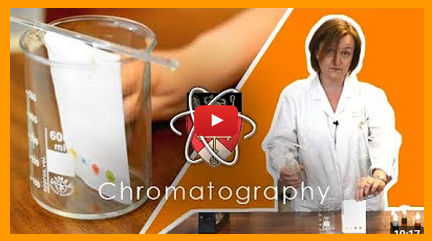
PRACTICAL - Chromatography
Paper chromatography is used to separate and identify substances in a mixture, such as dyes in ink.
In this practical, a small spot of ink is placed on a pencil line near the bottom of a piece of chromatography paper. The paper is then suspended in a solvent (like water), with the ink spot above the solvent level. As the solvent travels up the paper, it carries the different dyes at different rates, separating them into a pattern of spots.
PRACTICAL - Distillation
Distillation is used to separate a liquid from a solution by taking advantage of different boiling points.
In this practical, the solution is heated so the liquid with the lowest boiling point evaporates first. The vapour travels through a condenser, where it cools and condenses back into a liquid, which is then collected.
This method is used to purify liquids or separate components, such as getting pure water from saltwater.
Revision Notes

The Cornell method is like a supercharged note-taking system that helps you ace your revision!
Print out our blank revision notes pages to help you revise.
How to make effective revision notes with the Cornell method.
Why Do I Need to Know About Energy Changes?
In Everyday Life
- Understanding what’s in food, medicine, or makeup (e.g. elements and compounds)
- Reading and interpreting product labels with confidence
- Learning how radiation or nuclear energy works in the news
- Understanding how energy is stored or transferred (e.g. batteries, fuels)
- Making informed choices about health and the environment
In Science & Chemistry Careers
- Explaining chemical reactions at the atomic level
- Working with materials, energy, or nanotechnology
- Predicting how substances will behave using atomic and electronic structure
- Using atomic knowledge in fields like medicine, electronics, and energy
- Designing new elements or materials with specific properties